A Hybridized Orbital Can Contain Which of the Following
As we saw with N 2 the CO triple bond contains one sigma bond σ-bond because theres two sp-orbitals and two pi bonds π-bonds from both the carbons and the oxygens two 2p atomic orbitals-----Hybridization of BF 4-c. The remaining unhybridized p orbitals on the O atoms interact to form one pi bond.
Hybrid Atomic Orbitals Chemistry For Majors
The nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair.

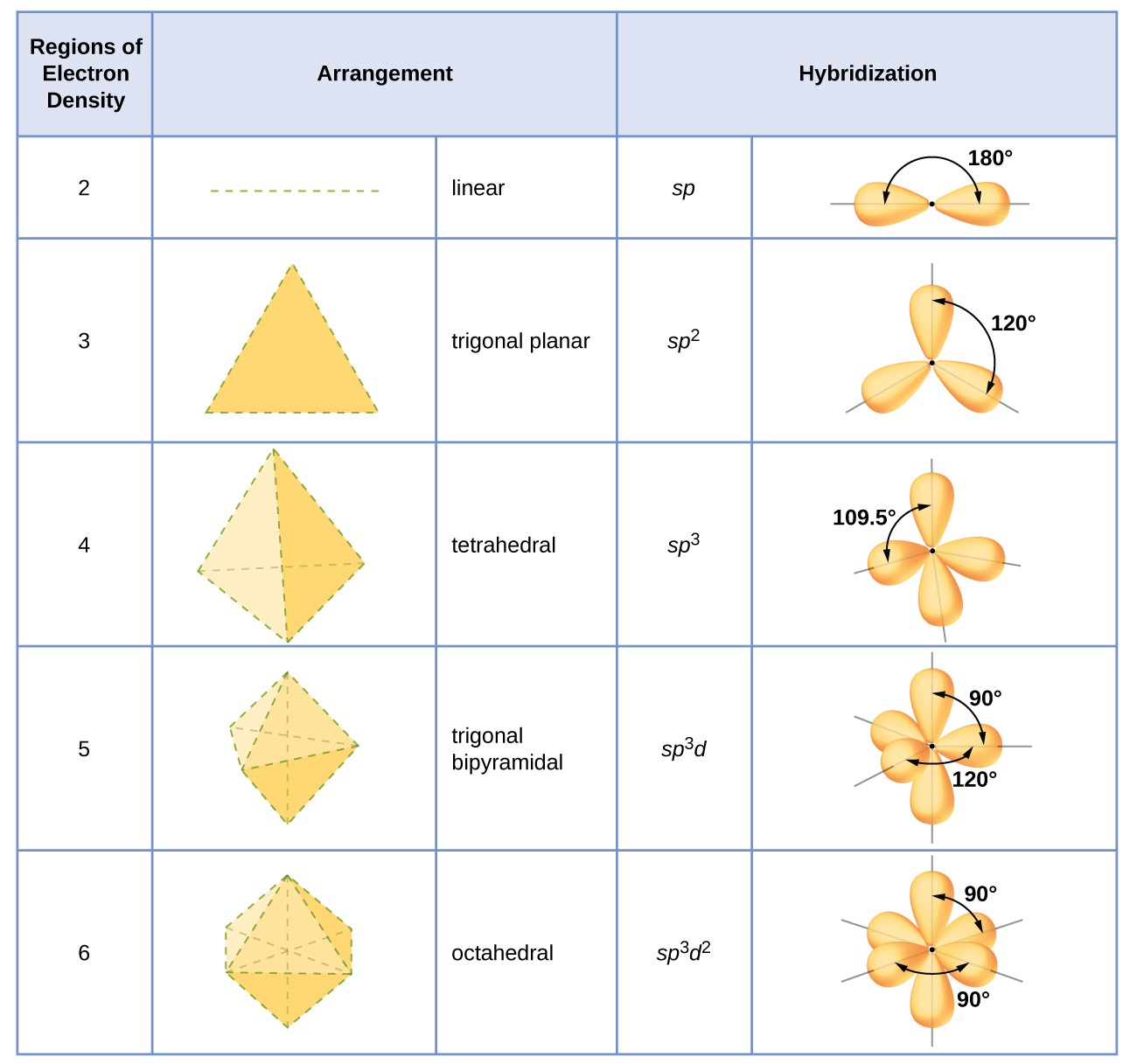
. An sp 3 hybrid orbital can also hold a lone pair of electrons. When hybridization occurs it must do so as a result of the mixing of nonequivalent orbitals. For general chemistry we just count the number of electron groups around the central atom and assume that the orbitals used are in order of angular momentum l.
In sp 3 hybridisation 1 s-oribital and 3 p orbitals are mixed to form 4 equivalent sp 3 orbitals. 2 sp3 orbitals require half of an s orbital and 15 p orbitals leaving 1 p orbital for one lone pair and half an s orbital and half a p orbital for the sp orbital the other lone pair to exist in. The singly occupied sp 2 hybrid orbitals on the O atoms interact to form a pi bond.
The hybrid atomic orbital model can be extended to molecules whose shapes are based on trigonal bipyramidal or octahedral distributions of electrons by including valence-shell d orbitals. It has 10 valence electrons and both the carbon and the oxygen are sp-hybridized. утсыг vvсир тигсрпс Dous Understand orbital overlap in multiple bonds Question A hybridized atomic orbital can contain or participate in which of the following.
Begingroup Sure you can. Pauling showed that when the 3 d z 2 orbital is mixed with the 3 s 3 p x 3 p y and 3 p z orbitals on an atom the resulting sp 3 d hybrid orbitals point toward the corners of a trigonal bipyramid. A hybridized atomic orbital can contain or participate in which of the following.
The molecular structure of water is consistent with a tetrahedral arrangement of. 6 C-H sigma σ bonds can be formed by the interaction of C-sp 3 with an H-1s orbital and 1 C-C sigma bond can be made by the interaction of C-sp 3 with another C-sp 3 orbital. Its Lewis Structure and its 3-D.
The nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. Each sp 3 hybrid orbital has 25 s character and 75 p character. Since lone pairs occupy.
The third p orbital remains an unhybridized atomic orbital. Formation of Ammonia NH 3 and Water H 2 O. The ion is trigonal planar with a H-C-H bond angle of 120.
The nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. This means that the orbital and the first orbital become a new orbital. The anion CH3- is sp3 hybridized hence only CH3 is sp2 hybridized among the options.
F e is s p 3 d 2 hybridized outer orbital complex having five unpaired electrons. The molecular structure of water is consistent with a tetrahedral arrangement of two lone pairs and two bonding pairs of electrons. O-bonds lone pairs of electrons single unpaired electrons radicals On-bonds FEEDBACK Content.
Ethane C 2 H 6 methane. Hence F e C l 6 3 is more paramagnetic than F e C N 8 3. The VSEPR model predicts geometries that are very close to those seen in real molecules.
The unhybridized atomic p orbital lies at a 90 angle to the plane. Sp2 hybridization involves the mixing of on s and two p orbitals. F e is d 2 s p 3 hybridized inner orbital complex having only one unpaired electron.
An sp 3 hybrid orbital can also hold a lone pair of electrons. By the interactions of C-sp 3 with an H-1s 4 equivalent C-H σ bonds can be formed. Hybrid orbitals are the result of a model which combines atomic orbitals on a single atom in ways that lead to a new set of orbitals that have geometries appropariate to form bonds in the directions predicted by the VSEPR model.
Orbital has one and one orbital hybridized. Sp 3 d Hybridization. One of the sp 2 hybrid orbitals on each O atom is singly occupied.
An atom can have both hybridized and unhybridized orbitals at the same time. Select all that apply. These are and.
NORMAL GENERAL CHEMISTRY WAY We assume an ordering of s p p p d d d d d f f f f f f f or sp3d5f7. This hybridization scheme is consistent with the Photoelectron spectrum data for water. So every sp 3 orbital contains 14 th of s-orbital and.
Chemistry questions and answers. The C C bond of ethyne H C C H has higher s character 50 than the C C bond of ethylene H X 2 C C H X 2 33 The hybrid atomic orbitals of the carbons of ethyne have a higher s character 50 than those of ethene 33 so the σ C H bonds will retain that property somewhat. Which of the following is named using the unmodified element name and adding the word ion.
Example of sp 3 hybridization. Thus we say that the oxygen atom is sp 3 hybridized with two of the hybrid orbitals occupied by lone pairs and two by bonding pairs. Up to 10 cash back This means that the one orbital can hybridize with 1 2 or all 3 orbitals.
Two of the sp 2 hybrid orbitals on each O atom contain a lone pair. Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. Hybridization is the mixing of the atomic orbitals in an atom to produce a set of hybrid orbitals.
They have trigonal bipyramidal geometry. This is observed in CH3. Because the three hybrid orbitals lie in one plane the VSEPR theory predicts that the orbitals are separated by 120 angles.
BF 4- has 32 valence electrons. For example the nitrogen atom in ammonia is surrounded by three bonding pairs and a lone pair of electrons directed to the four corners of a tetrahedron. This configuration allows for the maximum separation of all orbitals.
F e C N 6 3 has C N as strong ligand. Sp 3 d hybridization involves the mixing of 1s orbital 3p orbitals and 1d orbital to form 5 sp 3 d hybridized orbitals of equal energy. In NH 2 molecule the nitrogen atom.
For example the nitrogen atom in ammonia is surrounded by three bonding pairs and a lone pair of electrons directed to the four corners of a tetrahedron. In other words s and p orbitals can hybridize but p orbitals cannot hybridize with other p orbitals. Each O atom is sp 2 hybridized.
For example in NH_3 three N-H bonds are made and one lone pair of. Type of hybridization can be determined by the following steps given belowi Find the total number of valence electrons not valency. Since there are three total combinations there are three types of hybridized orbitals.
How Many Sp3 Hybrid Orbitals Result From The Hybridization Of S And P Orbitals Quora
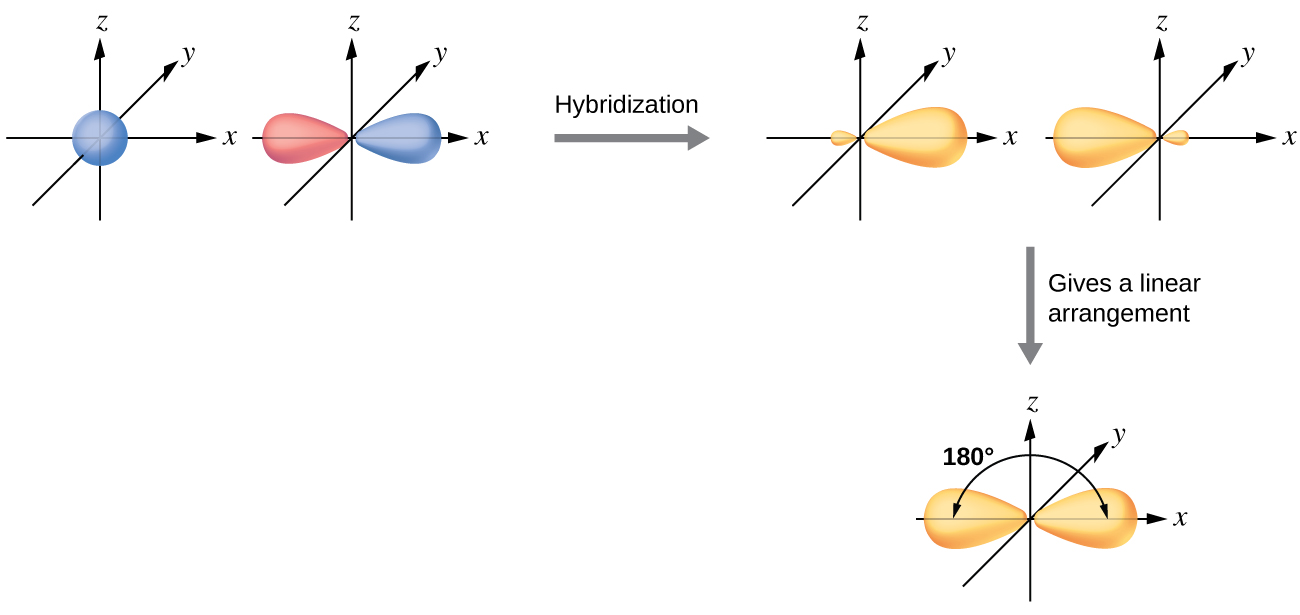
8 2 Hybrid Atomic Orbitals Chemistry

Hybridization In Molecules Containing Double Triple Bonds Video Lesson Transcript Study Com
No comments for "A Hybridized Orbital Can Contain Which of the Following"
Post a Comment